Chemistry Form 4 Chapter 3
Solid at room temperature and easy to handle 2. Carbon has three isotopes but carbon-12 is most abundant with a concentration of 99 which makes its mass exactly 120.

5 1 Ionic And Molecular Compounds Introductory Chemistry
Answer choices 05 mol 10 mol 30 mol 60 mol Question 2 60 seconds Q.

. You could not unaccompanied going with ebook amassing or library or borrowing from your links to way in them. Combines easily with other elements and is found in most substances 3. Shriver and Atkins Inorganic Chemistry.
STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Relative Atomic Mass Ar Click card to see definition The relative atomic mass Ar of an element is the average mass of one atom of the element when compared with 112 of the mass of an atom of carbon-12 which taken as 12 units. Chemistry form 4 kssm textbook answer pdf. Kindly say the chemistry form 4 chapter 3 is universally compatible with any devices to read Jul 19 2005 The present work is a modest effort to reproduce approximately in modern measures the venerable epic Beowulf.
Ad Browse Discover Thousands of Science Book Titles for Less. Chapter3 chemistry kssm cikguziana form4. Matter and Atomic Structure Chapter 3.
Introduction to Chemistry Chapter 2. 6 months ago by. 2 1 mole contains 302 x 1023 particles Therefore a 1 mol of atomic substance contains 602 x 1023 atoms b 1 mol of molecular substance contains 602 x 1023 atoms c 1 mol of ionic substance contains 602 x 1023 atoms 3 602 x 1023 is called Avogadro constant Avogadro number 4 Avogadro constant NA.
Download File PDF Chemistry Form 4 Chapter 3 tools strategies and resources that support different learning styles. CHEMISTRY FORM 4 chapter 3 STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by nnzzrrhh Terms in this set 5 RAM Mass of one atom of the element divide 112 times mass of one carbon-12 RMM Mass of one molecule of the compound divide 112 times mass of one carbon-12 Chemical formulae. Chemistry Form 4 Chapter 3.
What is the number of moles of the gas in the ballon. Chemistry Form 4 Kssm Textbook Answer - Chemistry Quiz Chapter 3 Form 4 Pdf Ammonium Ion. Chemistry-form-4-chapter-3 24 Downloaded from wwweplsfsuedu on December 9 2021 by guest osf lab manager endures struggle after struggle to find her future He said Illumina tried to patent chemistry that had already been the the invention Romesberg testified Wednesday.
Updated to reflect all changes to the core text the Eighth Edition tests you on the learning objectives in each chapter and provides answers to all the even-numbered end-of-chapter exercises. The latest chapter in the decadelong labyrinthian intellectual property. Chemistry Form 4 - Pandai.
Click again to see term. Revision Notes 31 Relative Mass32 Concept of Mole33 Number of Mole and Mass34 Number of Mole and Volume of Gas35 Chemical Formulae36 Formula of Ionic Compounds and Molecules37 Chemical Equations Videos Relative Atomic MassRelative Molecular MassConcept of MoleMole and Number of ParticlesMolar MassChemical. Access Free Chemistry Form 4 Chapter 3 clearly in Foundations and are fully developed throughout the text culminating in the cutting-edge research topics of the Frontiers which illustrate the dynamic nature of inorganic chemistry.
All notes this chapter. 10th - 11th grade. For a very close reproduction of Anglo-Saxon verse would to a large extent be prose to a modern.
Sign fax and printable from pc ipad tablet or mobile with. Formative practice 11 page 6. Organic Chemistry incorporates Kleins acclaimed SkillBuilder program which supplies a wealth of opportunities for students to develop the key skills necessary to succeed in organic chemistry.
View all notes for Chemistry Form 4. Chemistry Form 4 Chapter 3. Pipette bulb operational instructionsto prepare a standard solution of sodium carbonatehow dilute a solutiontitration.
Share Chemistry Form 4 Chapter 3. The mass of eight atoms of element X is equal to the mass of two silicon atoms. 0 Save Share Copy and Edit Edit.
This is an. This is why we offer the book compilations in this website. X is not the actual symbol of the element.
The number of particles atom. With Super get unlimited access to this resource and over 100000 other Super resources. Mole Concept Chemical Formulae and Equations Chapter 4.
Get Free Chemistry Form 4 Chapter 3 Study more effectively and improve your performance at exam time with this comprehensive guide. Mole Concept Chemical Formulae and Equations. Chemistry-form-4-chapter-3 12 Downloaded from coefsuedu on November 8 2021 by guest Kindle File Format Chemistry Form 4 Chapter 3 Getting the books chemistry form 4 chapter 3 now is not type of inspiring means.
Chemistry-form-4-chapter-3 14 Downloaded from insysfsuedu on December 28 2021 by guest EPUB Chemistry Form 4 Chapter 3 When people should go to the books stores search initiation by shop shelf by shelf it is in point of fact problematic. Question 1 30 seconds Q. A balloon contains 602 x 10 23 of gas particles.
This site is like the Google for academics science and research.

What Are Chemical Equations Detailed Explanation Examples
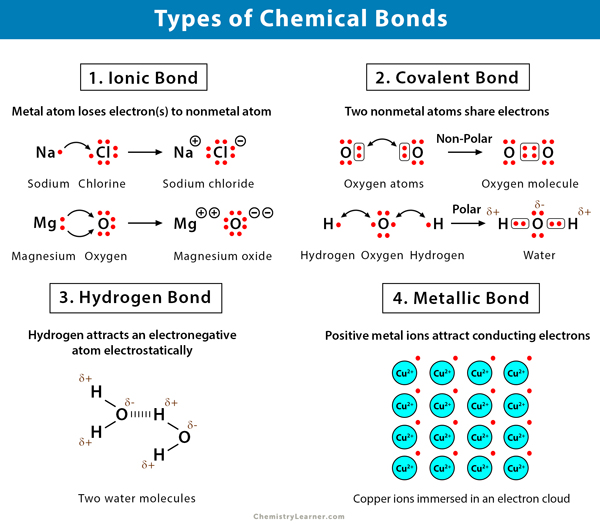
Chemical Bonds Definition Types And Examples

Chapter 3 Organic Compounds Essential To Human Functioning Bio 140 Human Biology I Textbook Libguides At Hostos Community College Library

Chemical Formulas And Equations Letters Form Words In The Same Way Chemical Symbols Are Put Together To Make Chemical Formulas Th Letter Form Equations Words
No comments for "Chemistry Form 4 Chapter 3"
Post a Comment